DRUG MANUFACTURING
We are leaders in the sector thanks to the wide range of pharmaceutical forms we offer (solids, semi-solids and liquids), our large production capacity, our highly qualified team and compliance with Good Manufacturing Practices (GMP) and national and international safety and environmental standards.
At Farmasierra Manufacturing, S.L. we have a properly organised Quality System, which ensures the strict monitoring of the established procedures and the quality of the products.
SEMI-SOLID FORMS
Gels and ointments in aluminium or roll-on tubes.
SOLID FORMS
Tablets, capsules, solution powder and powder for extemporaneous solutions.
LIQUID FORMS
Mixing and packaging-conditioning in solutions and suspensions manufacturing.
PHARMACEUTICAL FORMS MANUFACTURING
SEMISOLIDS
Within our manufacturing plant in San Sebastián de los Reyes we have a differentiated area for the manufacturing, packaging and conditioning of semi-solid forms with the highest quality standards:

GELS
This section is made up of 2 gel manufacturing zones, each equipped with 2,000/2,500 kg and 900 kg processors in ATEX anti-explosion facilities.

MANUFACTURING
This section is made up of 2 gel-manufacturing zones, each equipped with two 2000/2500 kg and 900 kg processors, as well as accumulation tanks, in ATEX anti-explosion facilities.

PACKAGING
Gel packaging is carried out in two independent, fully automated areas. We have an annual packaging capacity of 15 million tubes.

CONDITIONING
Inline conditioning allows standards: a high level of productivity.
OINTMENTS
Topical and ophthalmic ointments are prepared in two separate manufacturing, packaging and conditioning areas.

MANUFACTURING
We have 100 and 200 litre processors with an annual capacity of 40 tonnes. The transfer for packaging and conditioning is automated, maintaining the linear flow of manufacturing, which offers us great efficiency and productivity.

PACKAGING
Lines are properly equipped with quality control and machine vision devices to eliminate defective units.

CONDITIONING
Finally, the units are duly serialised in the final boxes and grouped on pallets to be sent to the finished product warehouse, and then dispatched to the distribution centres indicated by the customers.
PHARMACEUTICAL FORMS MANUFACTURING
SOLIDS
At the Farmasierra Group we have the facilities and machinery to manufacture various solid pharmaceutical forms with the highest quality standards and high production volume.

MIXING
In the mixing section we have various mixers, granulators, “single pot” unified systems for mixing, granulation, drying and mill connection systems to achieve particle size uniformity. The annual capacity is 1,100 tons.

MANUFACTURING
The different pharmaceutical forms, tablets, chewable tablets, coated tablets, hard gelatine capsules, powder for solution and powder for extemporaneous solutions are manufactured in this section in separate areas.

PACKAGE-CONDITIONING
The packaging is carried out in blister packs, bottles, packets and jars on automatic lines integrated with the serialisation systems of the packaging lines. The total annual production capacity is 16 million finished units in the different types of packaging.
DOSAGE FORMS MANUFACTURING
LIQUIDS
Our manufacturing plant in San Sebastián de los Reyes has different areas for the manufacturing, packaging and conditioning of liquid forms in solution or in suspension.

MANUFACTURING
The annual capacity for mixing liquids is 1.4 million litres for packaging in bottles in the form of solution or suspension in a wide range of formats.

PACKAGING
We have three high production packaging lines, equipped with advanced control systems.

CONDITIONING
The annual capacity is 12 million finished units, duly serialised and grouped on pallets which are transferred to the finished product warehouse.
CAPACITIES
SOLID FORMS
Tons of solid form mixing
Million Tablets
Million Coated Tablets
Million Hard Gelatine Capsules
Million Powder for solution packets
SEMI-SOLID FORMS
Tons of semi-solid mixtures
Million Tubes of gel
Million Tubes of ointment
LIQUID FORMS
Litres of liquid form mixing
Million packages
LEAN MANUFACTURING IN OUR
MANUFACTURING PLANT
Our pharmaceutical plant, located in San Sebastián de los Reyes (Madrid), has a total surface area of 16,000 m2 and a production area of 11,000 m2.
It has a warehouse for raw materials and materials, three parallel production sections for Semi-Solids, Solids and Liquids and a warehouse for finished products.
The manufacturing plant for pharmaceutical specialities is equipped with state-of-the-art technology and modern systems for communication with customers and suppliers, enabling us to provide the best service with the flexibility required by customers and the different markets.
Our production system follows the Lean Manufacturing methodology with a linear flow from the reception and storage of raw materials and materials, preparation of the components of each manufacturing batch, according to the approved formula, preparation of the pharmaceutical form, packaging, conditioning, storage of the finished product and dispatch of the finished product.
The pharmaceutical plant’s offices are located on the side of the building.
We have an area attached to the plant that provides purified water, steam, nitrogen and alcohol services, as well as our own high-capacity treatment plant.

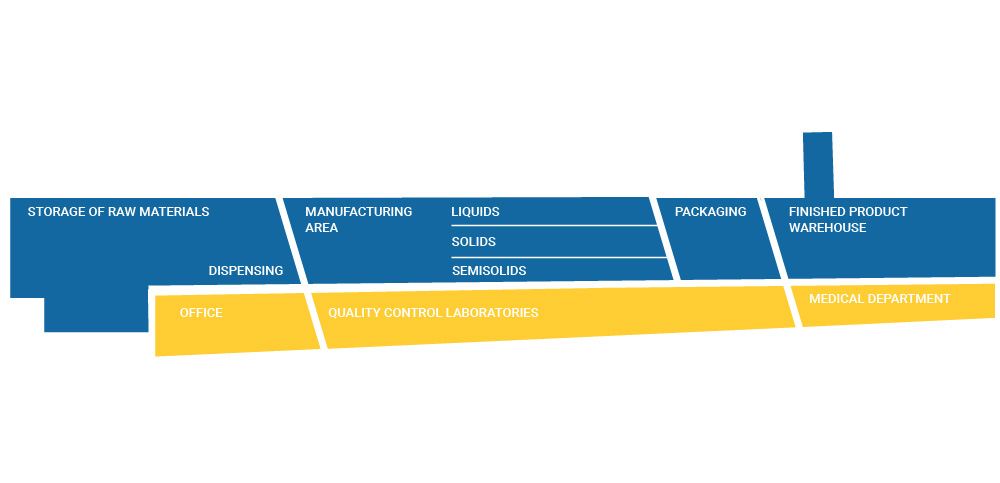

STORAGE OF RAW MATERIALS
It has 3 segregated areas for the storage of raw materials, packaging material, an area for rejected material, cold chambers, freezers and an archive area. The storage capacity is 3,700 pallets.

DISPENSING AREA
In the Dispensing Area, the components of the batches to be manufactured are prepared and weighed according to the established formulation and procedures, to be sent to the corresponding mixing areas. This area has a separate area for the storage and custody of active ingredients.

MANUFACTURING AREA
It has 3 segregated areas for the storage of raw materials, packaging material, an area for rejected material, cold chambers, freezers and an archive area. The storage capacity is 3,700 pallets.

FINISHED PRODUCT WAREHOUSE
With a capacity of 1,000 pallets and high rotation, we provide the service required by the client on the dates requested.
A long-term vision to find solutions for the future
INFORMATION TECHNOLOGIES
The Enterprise Resource Planning (ERP) system specialised in industrial environments is our backbone. All other systems are connected to it, and covers preventive, corrective and predictive maintenance operations and other more traditional systems such as Materials Requirement Planning (MRP) and connectivity with customers’ and suppliers’ supply chains that give us perfect visibility of the entire process.
The Laboratory Information Management System (LIMS) software from the Quality Control laboratory eliminates manual data entry and calculations, avoiding errors and making data more reliable. The Manufacturing Execution System (MES) for tasks in the manufacturing and conditioning areas eliminates the problem of paper in the pharmaceutical plant, achieving perfect traceability of operations in real time, as well as efficiency in operations, incidents and stoppages. The Dispensing system (weighing of the formula components) guarantees the perfect preparation of the raw materials.
CERTIFIED QUALITY
The Pharmaceutical Quality System (FQS) ensures the highest standards of product quality, guaranteeing compliance with the requirements of health authorities and specific customer requirements. The Pharmaceutical Quality System is continuously updated in accordance with the regulations of the health authorities of the various manufacturing destination countries.
At FARMASIERRA MANUFACTURING, S.L. we operate in compliance with Good Manufacturing Practices (GMP).
We are certified by the Spanish Agency of Medicines and Medical Devices (AEMPS), by the Food and Drug Administration of the USA (FDA), by the Brazilian health authorities ANVISA, as well as from other countries.
We are also authorized and certified for the Preparation of Medication for Clinical Trials (Investigational Medicinal Products, IMP).
We use an Environmental Management System that meets the requirements of ISO 14001, and are also certified by the British Standards Institution (BSI).
The Quality Team includes the Pharmaceutical Technical Management, Quality Control, Quality Assurance and Validation laboratories, Archive and Climate Chamber Area, to which duly trained and specialised teams are assigned.
